
Therefore, there are various non-equivalent definitions of atomic radius.
DENSITY OF KRYPTON FREE
However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. It must be noted, atoms lack a well-defined outer boundary. The atomic radius of Krypton atom is 116pm (covalent radius). The krypton element symbol is Kr, its atomic number is 36, and its atomic mass is 83.798. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. Krypton is a chemical element found in Group 18 of the periodic table (i.e., the noble gases). In this work we use density functional theory to model the diffusivity of matrix U. About three times heavier than air, krypton is colourless, odourless, tasteless, and monatomic. The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. Uranium mononitride is a strong candidate for an advanced nuclear fuel. krypton (Kr), chemical element, a rare gas of Group 18 ( noble gases) of the periodic table, which forms relatively few chemical compounds. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. Mass numbers of typical isotopes of Krypton are 80 82-84 86. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z.įor stable elements, there is usually a variety of stable isotopes. Neutron number plus atomic number equals atomic mass number: N+Z=A. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. Areas covered include atomic structure, physical properties, atomic interaction, thermodynamics.
DENSITY OF KRYPTON FULL
Each entry has a full citation identifying its source.

Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Our krypton page has over 220 facts that span 73 different quantities. Where more than one isotope exists, the value given is the abundance weighted average. This is approximately the sum of the number of protons and neutrons in the nucleus.
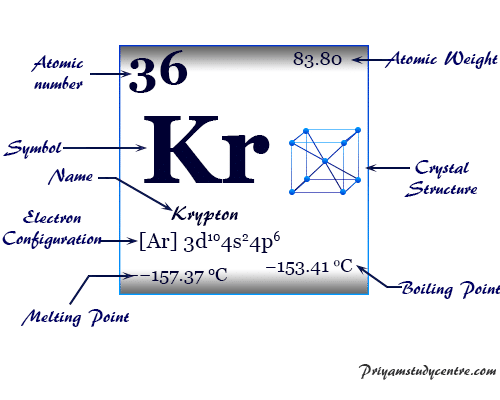
Relative atomic mass The mass of an atom relative to that of carbon-12. Krypton is a chemical element with atomic number 36 which means there are 36 protons in its nucleus. Density is the mass of a substance that would fill 1 cm3 at room temperature. Density is defined as the mass per unit volume. Atomic Number – Protons, Electrons and Neutrons in Krypton The ionization degree and equation of state of dense krypton have been calculated in the range of temperature of 4-50 kK and density of 0.01-10 g/cm 3. Typical densities of various substances are at atmospheric pressure.


 0 kommentar(er)
0 kommentar(er)
